Introduction
Imagine you’re testing a hospital management application used in a busy emergency-ward setting. A nurse reports that the patient ID field accepts alphanumeric characters when only numeric IDs should be allowed. That’s a defect. The journey of this defect from discovery, logging, assignment, fix, retest to closure is the defect life cycle. In any QA project, especially where stakes are high (like healthcare software), a well-defined defect life cycle ensures transparency, accountability, and quality. In fact, research shows that tracking defects early lowers cost and risk. When you take Quality assurance certification courses, live projects will often simulate or use actual defect life-cycles. Understanding what these look like in real-time helps you bridge theory into practice.
Defining the Defect Life Cycle
What is it?
The defect life cycle (also called bug life cycle) refers to the series of stages a defect goes through from its detection to its closure.
It helps multiple stakeholders, testers, developers, QA leads track where a defect stands, what actions remain, and who is responsible.
Why it matters in QA
- It improves communication: status is clear, and roles are defined.
- It helps prioritise: teams can classify defects by severity, impact, and risk.
- It supports compliance and audit trails: especially in regulated domains like healthcare, you need a documented trail of how defects were handled.
- It contributes to cost-control: early detection and management of defects reduces rework cost.
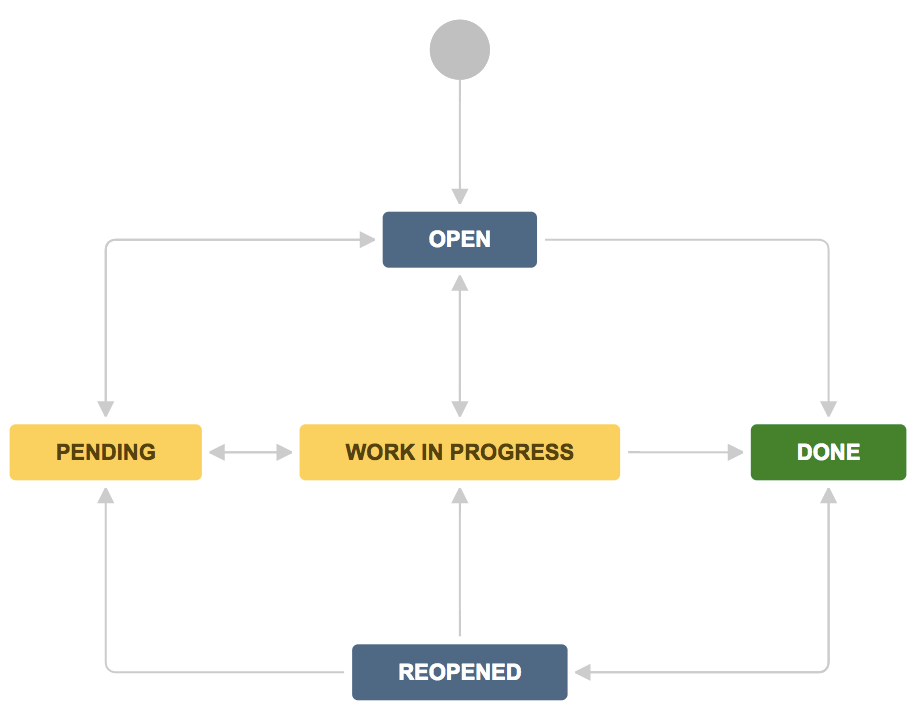
Typical States of the Defect Life Cycle
Most projects use a similar set of states, though names and number of states may vary. Here’s a commonly used list:
- New: Defect is identified and logged.
- Assigned: It is assigned to a developer or team.
- Active / In Progress / Open: Developer investigates, reproduces, or begins fix.
- Fixed / Resolved / Ready for Retest: Developer marks issue as fixed and passes to test team.
- Pending Retest / Retest: Tester re-executes tests to verify fix.
- Verified / Closed: Tester confirms resolution and marks defect closed.
- Reopened: If the test fails, the defect is reopened and goes back into investigation/fixing.
- Deferred / Rejected / Duplicate / Not a Bug: Alternate statuses when defect is postponed, invalid, duplicate or out-of-scope.
Here’s a simple diagram to visualise it:
Real-Time QA Project Example – Web Application
Let’s walk through a concrete example from a live project scenario for a web-based e-commerce platform. This is similar to what you might handle in a quality assurance certification course’s live project module.
Project background
The QA team is testing an e-commerce website module for order checkout. One of the test cases: “When a user applies a valid coupon code, discount should apply and total amount recalculated”.
Defect life cycle example
- Detection / New:
The tester executes the test case, enters a valid coupon code but sees no discount applied. Tester logs the defect: “Coupon code applied but total amount unchanged”. Build version 1.3.0. - Assigned:
The defect report gets reviewed by QA lead and assigned to the developer responsible for the checkout module. Status changes to “Assigned”. - Open / Active:
Developer investigates problem: reproduces the issue, finds that coupon validation API returns correct status but the front-end function to recalc total is missing. The developer logs the root cause and begins to fix. Status: “In Progress”. - Fixed:
Developer updates front-end code to call correct recalc function, adds unit tests, pushes build 1.3.1. Marks defect status “Fixed”. - Pending Retest:
The fixed build is handed back to QA for retesting; status “Pending Retest”. - Retest:
The tester runs the same test case: coupon code applied, discount shows, total recalculates correctly. The tester also checks boundary cases (expired coupon, 0 discount, combination with gift-card). All pass. Status: “Retest” → then “Verified”. - Closed:
Tester marks defect status as “Closed”, signifying it is resolved and no longer reproducible. - If Reopened:
Suppose later a user reports that combining coupon and gift-card fails in production. The QA lead may reopen defects, status becomes “Reopened” and cycle repeats.
Why this example matters in live projects
- The sequence shows clear transitions and ownership.
- It emphasises retesting and validation, not just code fix.
- It maps to training you get in quality assurance courses online by simulating realistic workflows.
- It demonstrates the importance of version control, environments, and tracking build numbers common in quality assurance certification courses.
Hands-on tip (for your live project work or QA certification):
When logging defects, use a template with fields like: Defect ID, Summary, Steps to Reproduce, Expected vs Actual, Severity, Priority, Environment, Build version, Assigned to, Status. Many platforms like Jira or Bugzilla support this.
Real-Time QA Project Example – Healthcare Domain
Now let’s see an example from the healthcare domain very relevant if you look at quality assurance courses in healthcare. Healthcare software demands extra care because defects can impact patient safety, regulatory compliance, and data security.
Project background
The project is a hospital information system (HIS). One module: “Patient admission/discharge summary.” Business requirement: When a patient is discharged, the system must auto-generate a discharge summary within 5 minutes and notify the doctor for signature.
Defect life cycle example
- New:
QA tester notices that after discharge, the summary is delayed by 15 minutes, and no notification is sent. Logs defect: “Delayed discharge summary generation and missing notification”. Severity = High (because patient discharge flow is disrupted). - Assigned:
QA lead assigns defect to the HIS team developer; status changes to “Assigned”. - Active / In Progress:
The developer investigates. They find that the summary job scheduler failed because of a mis-configured cron job. Developer corrects scheduler settings, adds alert logic. Status: “Fixed”. - Pending Retest:
Build deployed to QA staging; tester prepares test data like discharge flows, multiple patients in parallel to check concurrency. Status: “Pending Retest”. - Retest:
Tester runs scenario: Patient A discharge → summary generated within 2 minutes, notification sent; Patient B simultaneous discharge → summary in 4 minutes. All pass. Status: “Verified”. - Closed:
Because of the severity and regulatory audit requirement, documentation is attached: root-cause, fix details, retrospective meeting minutes. Defect status: “Closed”. - Deferred or Reopened:
If the fix works in staging but after rollout to production, system delay occurs for very high load, the defect may get reopened or flagged for further improvement (Deferred).
Key considerations for healthcare QA
- Regulatory traceability: You may need audit logs showing defect resolution.
- Impact on patient care: High severity demands immediate attention; life cycle must support escalations.
- Data privacy/security: Some defects may relate to PII or medical data; hence additional states like “Under Security Review” may appear.
- High-stakes retesting: You may need to re-validate in production-like environments.
By working through this example you appreciate how defect life cycle maps to real-time QA in healthcare, reinforcing why quality assurance certification courses emphasise these workflows.
Common Variations & Tool Support
Variations in real-time projects
While the core cycle remains similar, live projects often adapt the life cycle to their methodologies (e.g., Agile vs Waterfall), regulatory needs, or organisational norms.
- Some teams merge “Assigned” and “Open” into “In Progress”.
- Some add states like “On Hold” or “Awaiting Info” when more data is needed.
- Some use “Deferred” for defects scheduled for future release.
- The number of states and their names vary across projects and tools.
Tool support
Defect tracking tools are critical. Common ones include Jira, Bugzilla, Redmine. They allow you to:
- Log defects with custom fields (severity, priority, environment)
- Assign to users and change status workflows
- Generate dashboards and metrics (e.g., number of open defects by severity)
- Link to test cases, requirements, versions
Metrics & monitoring
Live projects often track metrics such as:
- Time to close defects (from New → Closed)
- Defect density (defects per KLOC or per module)
- Re-opened defect count (quality of fix)
- Severity distribution (how many Critical, Major, Minor)
Research shows that organisations with mature defect-life-cycle tracking achieve better outcomes. For example, a case-study combining expert judgment and defect data found improved estimation accuracy.
Diagram: Tool workflow example
Practical Steps for QA Learners & Certification Projects
If you’re enrolled in quality assurance courses online or pursuing quality assurance certification courses (particularly those that include live projects), here’s how you can apply the defect life cycle knowledge practically:
- Set up your defect tracking template
- Define fields: ID, Summary, Description, Steps to Reproduce, Environment, Severity, Priority, Status, Assigned To, Build version, Reporter, Dates.
- Choose or simulate a tool (can be Excel when practising, or Jira if available).
- Practice a mini-project
- Take a small module (e.g., login, payment, patient registration) and design test cases.
- Execute tests, intentionally seed defects (or use realistic ones), log them, and move them through full life cycle.
- Retest fixes, update status, close.
- Map states to real-time project
- When training in a live project module, ask: What are our defect life cycle states? Are they labelled “New, Open, Resolved”? Or “New, Assigned, In Progress, Fixed, Ready for Retest, Closed”?
- Understand how your tool handles status transitions, branching (e.g., Rejected, Duplicate) and escalation.
- Use severity & priority correctly
- Severity: impact on system (e.g., system crash vs cosmetic issue).
- Priority: how soon to fix (e.g., high for release-blocking).
- Document these in your defect reports. This practice is often emphasised in certification courses.
- Track metrics and retrospective
- At the end of live project iterations, gather metrics: open vs closed defects, average time to fix, number reopened.
- Reflect: Are we catching many defects late? Are fixes causing re-opens? Use that insight to improve.
- In healthcare or regulated domains, ensure audit trails and approval flows are captured.
- Link to requirements & test cases
- In your live project, ensure defects are traceable back to test case and requirement. This is often a requirement in QA certification.
- Helps in root-cause analysis and prevents similar defects in future cycles.
Code snippet / pseudo-script for defect logging (if using automation)
def log_defect(test_case_id, build_version, steps, expected, actual, severity, priority):
defect = {
“TestCaseID”: test_case_id,
“Build”: build_version,
“StepsToReproduce”: steps,
“ExpectedResult”: expected,
“ActualResult”: actual,
“Severity”: severity,
“Priority”: priority,
“Status”: “New”
}
# This could call an API for Jira/Bugzilla
defect_id = defect_tracker_api.create(defect)
print(f”Defect logged: {defect_id}”)
return defect_id
This snippet shows how automation/test-framework code could integrate with defect life cycle logging useful for live project work and for hands-on modules in QA certification courses.
Advanced Example Agile Sprint with Continuous Deployment
In modern QA projects (including live projects in certification programs), teams often work in Agile and use continuous deployment. Let’s illustrate the defect life cycle in that context.
Setting
Team uses Scrum, two-week sprints, deploys to staging every 3 days, and to production at end-of-sprint. They use continuous integration and automated tests for regression. A defective life cycle needs to keep pace with this speed.
Example flow
- New / Found during sprint
Tester finds a UI misalignment in mobile view of checkout page severity Medium. Logs defect as “New”. - Triage / Assigned same day
Daily stand-up: developer takes up defect this sprint, marks status “Assigned”. - Active / In Progress
Developer fixes CSS and mobile breakpoint issue, commits to branch, CI auto-runs, passes, build 1.4.2 is created. - Fixed / Ready for Retest
The defect is marked “Fixed”; tester is notified via tool. - Retest / Verified
Tester executes on multiple devices, verifies fix, updates status to “Verified”. - Closed
Because the sprint ends soon and the fix is verified, the defect is closed. - Deferred for next release
Meanwhile another defect (performance lag under >500 concurrent users) is found, labelled severity High but the developer team opts to fix in next sprint due to resource constraints status is set “Deferred”.
Key takeaways for live project learners
- The life cycle must be agile-friendly: fast transitions, continuous updates.
- Tooling and automation helps integrate defect life cycle into CI/CD pipeline.
- Learners in QA certification courses need to be comfortable with status updates, dashboards, and reporting.
- Use dashboards to show sprint defect trends: e.g., “Open at sprint start”, “Closed during sprint”, “Carried over to next sprint”.
Specific Considerations for Healthcare QA Projects
When you specialize in quality assurance courses in healthcare, the defect life cycle takes on additional dimensions. Let’s review these key considerations.
Regulatory and compliance aspects
- Healthcare software often must comply with standards like ISO 13485 (for medical devices) or HL7 interoperability standards. Defects might have regulatory implications thus tracking & documentation become critical.
- Defect logs may need to include risk classification (e.g., patient safety risk), audit trail (who changed status, when), and validation evidence.
- Status states may include “Under Compliance Review” or “Risk Assessment Pending”.
High severity & escalation
- A defect in a healthcare system can lead to patient mis-treatment. Thus severity thresholds are tighter and escalation paths faster.
- The defect life cycle may integrate an “Escalated” state or an emergency fix path.
Traceability & retrospective
- After closure, there might be mandatory root-cause analysis, retrospective meetings, and preventive actions. This continues the quality assurance process beyond just closure of defects.
- The defect life cycle may feed into continuous improvement: e.g., identification of common defects leads to process change.
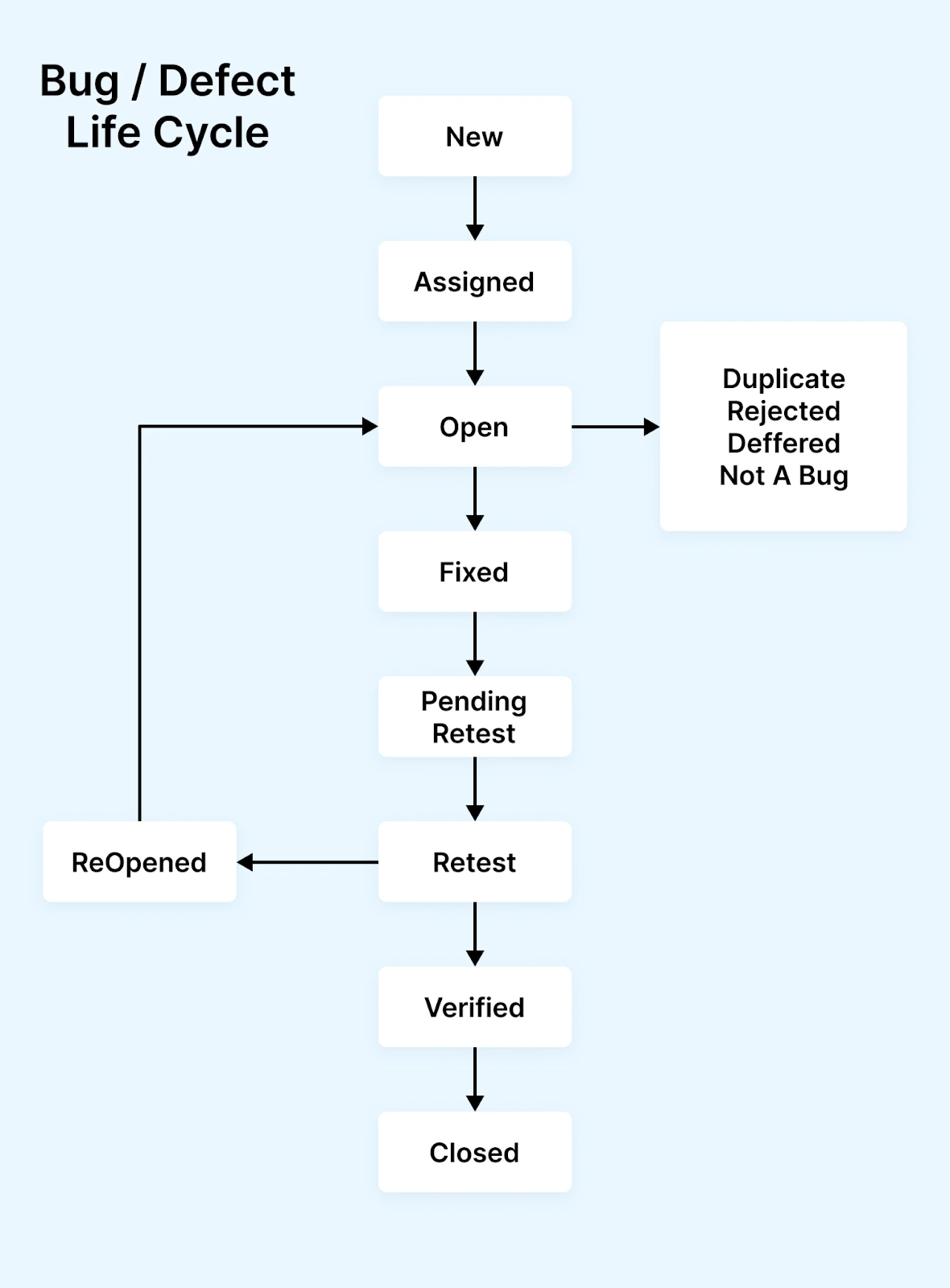
Example: Healthcare live project scenario
Defect: “Patient allergy alert not popping up during medication prescription for discharged patient.”
- New → Assigned → In Progress → Fixed → Pending Retest → Retest → Verified → Closed
- Additional steps: Risk classification (High), compliance review before production release, monitoring in production for next 24 hrs with alert active.
Visual: Healthcare defect life cycle flow
For learners doing live projects in healthcare QA certification, these extra layers are essential to understand and practise.
Common Mistakes & Best Practices
Common Mistakes
- Logging defects without clear reproduction steps, which leads to delays.
- Using unclear statuses or inconsistent workflows leads to tracking confusion.
- Not retesting thoroughly, the defect gets closed prematurely and reopens later.
- Ignoring metrics or ignoring root-cause analysis misses opportunities for process improvement.
Best Practices
- Define and document the defect life cycle workflow at project start: states, transitions, roles.
- Standardise defect fields: severity, priority, reproducibility, steps, environment.
- Use tool automation: notifications, dashboards, linking to test cases & requirements.
- Retest thoroughly: include regression tests to ensure the fix didn’t break other areas.
- Track metrics: open vs closed, time to close, reopened rates use in retrospectives.
- Link defects to improvements: after closure, ask “Why did this happen?”, “What process change can prevent recurrence?”
In your quality assurance certification courses, you’re often asked to both perform live projects and reflect on these practices so understanding mistakes and best practices adds value.
How This Maps to Quality Assurance Certification & Courses
When you enrol in quality assurance certification courses, especially those with live project components, the defect life cycle is central to your learning. Here’s how:
- Theory modules introduce defect life cycle: states, workflows, metrics.
- Hands-on labs: You log defects, move them through status, perform retests, close them, log metrics.
- Live projects: You simulate or work with real modules (web, mobile, healthcare) and manage defects end-to-end.
- Assessments: You may be evaluated on how well you adhere to the defect life cycle, the clarity of defect reports, the analysis of data/metrics.
- Specialisation: In courses focusing on quality assurance courses in healthcare, you’ll encounter healthcare domain-specific defect workflows and regulatory compliance considerations.
- Online delivery: Many quality assurance courses online provide video lecture, quiz, lab and project dashboards ensuring you get real world exposure to defect life cycles.
By mastering the defect life cycle in these courses, you build industry-relevant skills: defect logging, tracking, retesting, tool usage, metrics, communication across teams which hiring managers value highly.
Conclusion
The defect life cycle is more than a process; it’s the backbone of how you manage quality in software and systems, especially when working on live projects through quality assurance certification courses. Whether you’re working on a web app, a mobile product, or a healthcare system, knowing how a defect moves from discovery through closure, and handling that journey properly, sets you apart.
When you practise in quality assurance courses online or enrol in quality assurance certification courses, make sure you engage deeply with real-time defect life cycles log defects, move them through states, retest, close, measure. In the healthcare domain, always keep safety, traceability, and audits in mind.
Key Takeaways
- The defect life cycle tracks a defect from discovery to closure; clear states improve transparency.
- Real-time QA projects (e-commerce, healthcare) reinforce the importance of good defect tracking, clear reporting, and tool usage.
- In healthcare QA, defect life cycle must account for regulatory, safety, and traceability concerns.
- Hands-on experience (logging defects, using tools, metrics) in certification courses helps you gain practical skills.
- Avoid common mistakes (poor reports, inconsistent workflows), and adopt best practices (documentation, retesting, metrics, root-cause).